Regulatory Unit for medical devices and in vitro diagnostic devices
The regulatory support unit of the Laboratory of Automatics is a global consulting unit specializing exclusively in the medical device (MD) and in vitro diagnostic device (IVD) sectors and has expertise in helping clients achieve global compliance in multiple markets, including Europe, the United States of America, Brazil, among others. The team is composed of consultants specialized in different areas to provide global support to clients even if recently entered the world of health products.
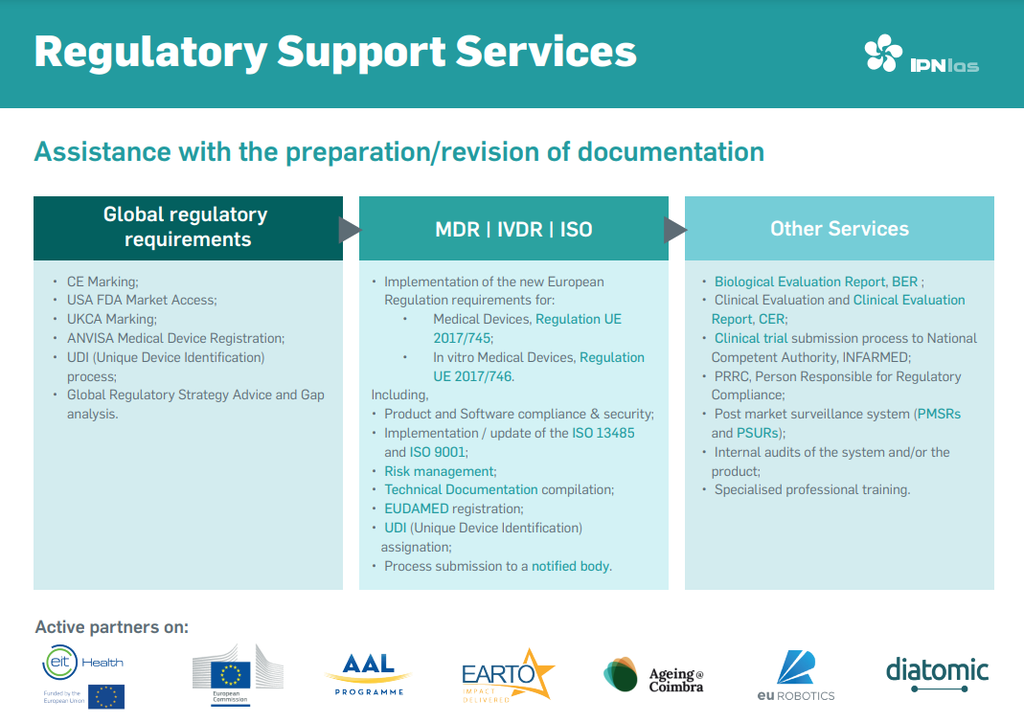
Services provided include: Support in implementing the requirements of the new European Regulation applied to Medical Devices, Regulation (EU) 2017/745;
Support in implementing the requirements of the new European Regulation applied to in vitro Medical Devices, Regulation (EU) 2017/746;
Support in the UDI (Unique Device Identification) assignation process;
Support in registering on the EUDAMED database;
Support in the complete CE Marking process;
Support in the ANVISA process;
Support in the FDA clearance process;
Support in the UK certification process;
Support in implementing/updating ISO 13485 and ISO 9001;
Biological Evaluation Report (BER);
Clinical Evaluation Report (CER) and Performance Evaluation Report (PER);
National entities licensing;
Indication of entities certified for testing, in particular by ISO 60601;
Support in submitting the process for certification by a Notified Body;
Support in the clinical trial submission process to INFARMED, through preparation or review of documentation and submission to RNEC;
Person Responsible for Regulatory Compliance;
Vigilance of the market;
Elaboration or revision of PMSR and PSUR reports;
Internal audits of the system and/or product;
Specialized consulting hours;
Specialized professional training.